i. Hydrogen + Chlorine → Hydrogen chloride
ii. Barium chloride + Aluminium sulphate → Barium sulphate + Aluminium chloride
iii. Sodium + water → Sodium hydroxide + water
iv. Sodium hydroxide solution (in water) reacts with hydrochloric acid solution (in water) to produce sodium chloride solution and water.
v. Calcium hydroxide + Carbon dioxide → Calcium carbonate + Water
vi. Zinc + Silver nitrate → Zinc nitrate + Silver
vii. Aluminum + Copper chloride → Aluminum chloride +Copper
viii. Barium chloride + Potassium sulphate → Barium sulphate + potassium chloride
ix. Potassium bromide (s) + Barium iodide (aq) → Potassium iodide (aq) + Barium bromide(s)
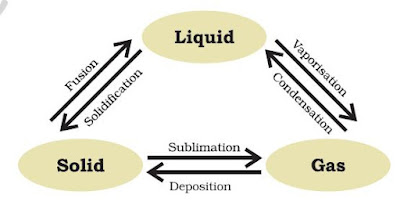
x. Zinc carbonate (s) → Zinc oxide (s) + Carbon dioxide (g)
xi. Magnesium (s) + Hydrochloric acid (aq) → Magnesium chloride (aq) + Hydrogen (g)
xii. Iron + Steam → Iron Oxide + Hydrogen
xiii. Silver bromide → Silver metal Bromine
xiv. Hydrogen peroxide→ Water + Oxygen
xv. Iron + Copper sulphate → Iron (II) sulphate + Copper
xvi. Zinc + Copper sulphate → Zinc sulphate + Copper
xvii. Lead + Copper chloride→ Lead chloride+ Copper
xviii. Potassium iodide + Chlorine → Potassium chloride Iodine
xix. Silver nitrate+Sodium chloride → Silver chloride+ Sodium nitrate
xx. Copper (Brown) +Oxygen→ Copper (II) oxide (Black)
xxi. Calciumoxide + Water → Calcium hydroxide
xxii. Sodium carbonate + Calcium chloride → Calcium carbonate + Sodium chloride
xxiii. Aluminium+ Copper chloride → Aluminiumchloride +Copper
xxiv. Zinc carbonate(s) → Zinc oxide(s) + Carbon dioxide(g)
xxv. Magnesium(s) + Hydrochloric acid(aq → Magnesium chloride(aq) + Hydrogen(g)
xxvi. Ferrous sulphate → Ferric oxide + Sulphur ioxide+ Sulphur trioxide
xxvii. Silver chloride → Silver + Chlorine
xxviii. Aluminium oxide → Aluminium + Oxygen
xxix. Silver nitrate+ Copper → Copper nitrate+ Silver
xxx. Sodium sulphate+ Barium chloride → Barium sulphate + Sodium chloride